LockBody® Technology Platform
Aiming to redefine immuno-oncology treatment for patients with cancer
Our team of scientists at Centessa are continuously inspired by both professional and personal experiences surrounding the unmet needs in cancer therapy, particularly the gap in cancer treatments that are both efficacious and safe. Our proprietary LockBody® technology platform hopes to tackle these unmet needs.
LockBody® drug candidates are designed to selectively drive potent effector function activity, such as CD47 or CD3, to the tumor microenvironment while avoiding systemic toxicity.
Novel MOA
LOCKED CONFIGURATION
outside the tumor microenvironment
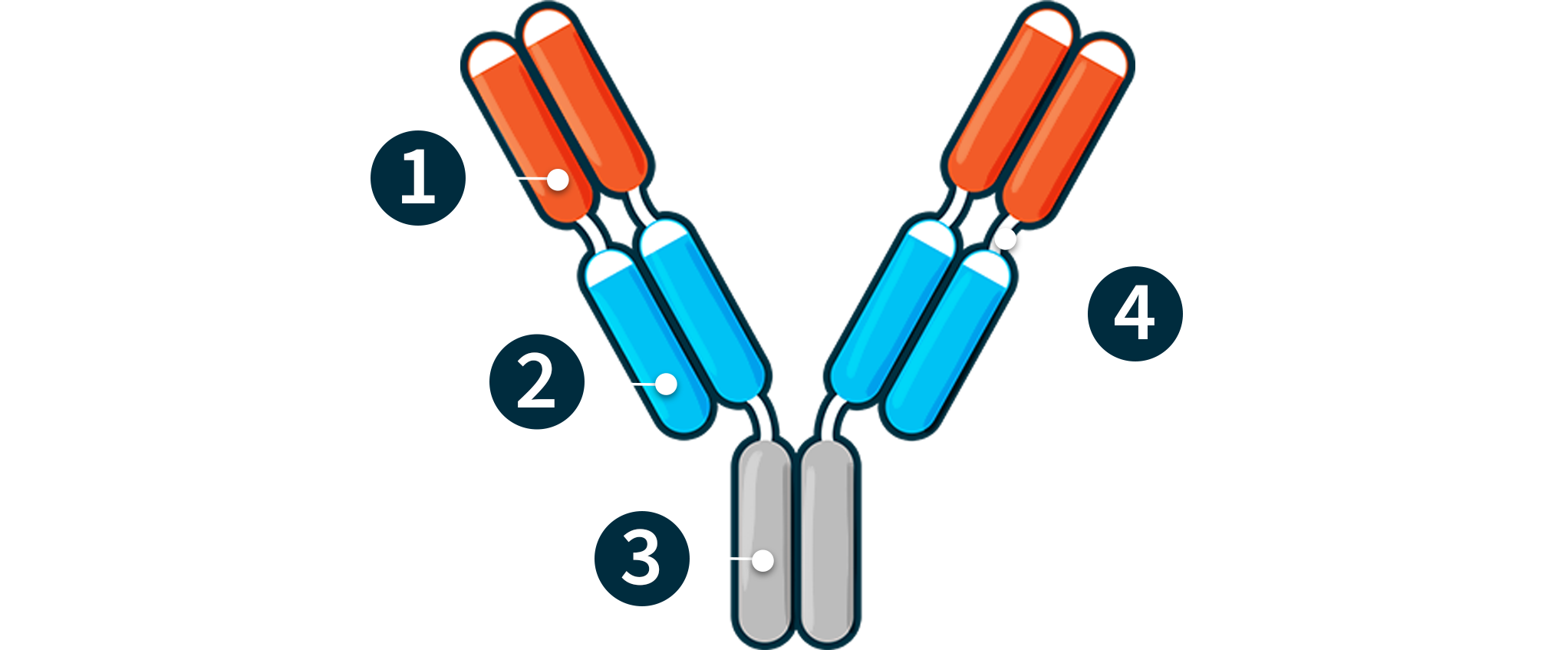
Constitutive Fabs – For targets expressed in diseased tissues (e.g., PD-L1); drive tumor enrichment
Locked Contingent Fabs – For tumor or immune cell targets; remain blocked by contitutive Fabs (e.g., CD47; CD3)
Fc Region
IgG1-derived Hinges – Naturally resistant to cleavage in serum


UNLOCKED CONFIGURATION
inside the tumor microenvironment

Unlocked Contingent Fabs – Activate effector function activity in the tumor microenvironment (e.g., CD47; CD3)
Exposed CDRs
IgG1-derived Hinges – Susceptible to cleavage in diseased tissue by various natural processes

Our LockBody® antibodies are designed to:



Avoid the ‘sink effect’
Stability of the LockBody® in the periphery minimizes unwanted binding to effector targets highly expressed in the blood and secondary lymphoid tissue. This design has the potential to lower effective doses and mitigate the risk of toxicity.
Enable a potentially dramatic enhanced therapeutic index
To fully activate LockBody®, two criteria are needed: the presence of an appropriate binding target of the constitutively-active upper Fabs and a highly proteolytic environment enriched with effector function. This dual requirement may potentially widen the therapeutic index and provide access to potent effector mechanisms.
Support high manufacturing yields and low immunogenicity
As all the components of a LockBody® are based on native, human IgG structures they have the potential to retain all the key benefits of standard therapeutic antibodies including high manufacturing yields and low immunogenicity.
Drug Candidate
LB101
Investigational, novel, conditionally tetravalent PD-L1 X CD47 bispecific monoclonal antibody, designed to be a single-agent systemic treatment in PD-1/PD-L1 refractory and resistant settings.
At Centessa, we are exploring additional LockBody molecules.
Ongoing Clinical Study
LB101 is an investigational agent that has not been approved by the FDA or any other regulatory authority.
LB101 is currently in Phase 1/2 first-in-human trial for the treatment of advanced solid tumors.
NCT05821777: LB101 in advanced solid tumors
For additional detail, please visit www.clinicaltrials.gov (US), www.clinicaltrialsregister.eu (EU), or registries in other jurisdictions.