Orexin Receptor 2 Agonist Program for Sleep-Wake Disorders
A potential best-in-class treatment for sleep-wake disorders
Orexin agonists have the potential to address the underlying cause of narcolepsy type 1 (NT1) and bring potentially transformative treatment options to enhance patient lives in NT1, narcolepsy type 2 (NT2), idiopathic hypersomnia (IH) and other potential sleep-wake disorders. However, there exists a significant gap in the development of orexin agonists, attributed to their complex medicinal chemistry. At Centessa, we aim to overcome this obstacle by leveraging structural biology techniques and structure-based drug design to help guide the design of small molecule orexin agonists.
We aim to address these unmet needs with our lead orexin agonist drug candidate, ORX750.
Drug Candidate
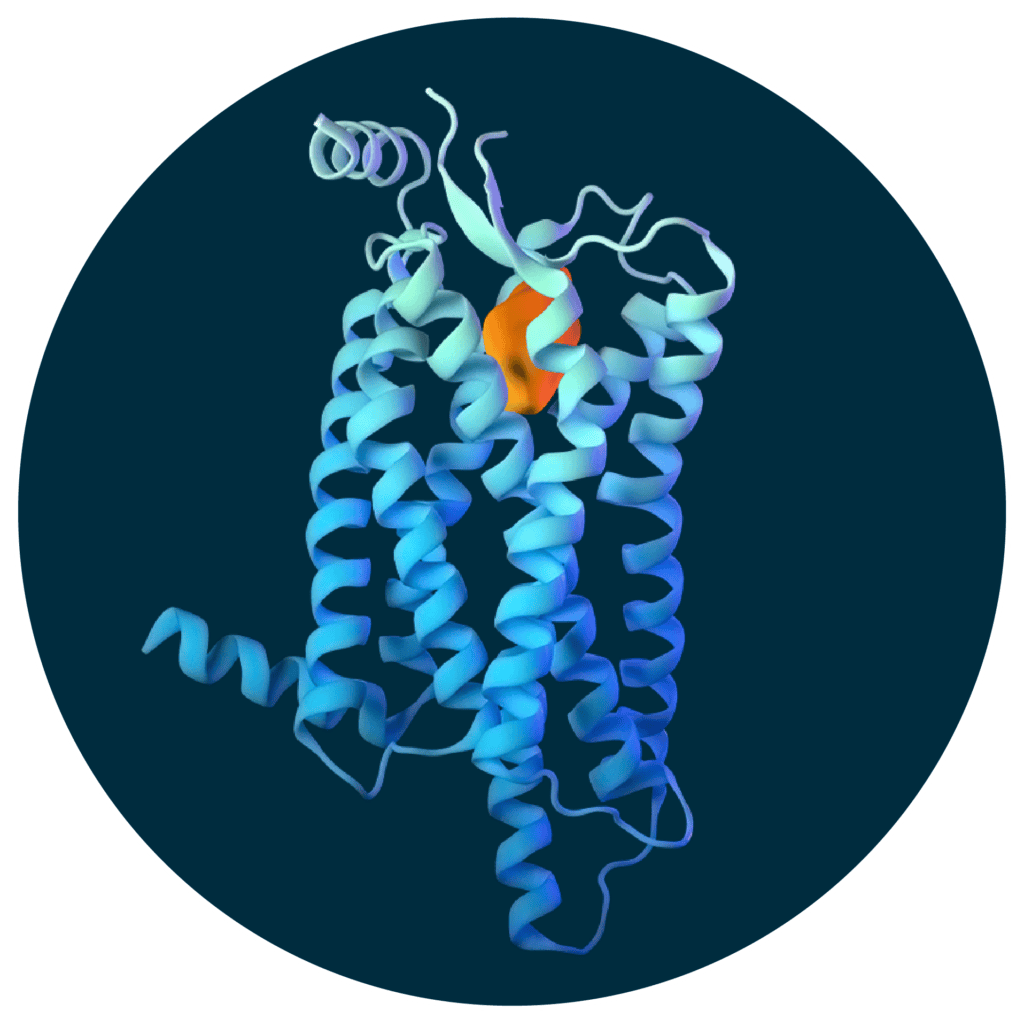
ORX750 is an investigational orally administered, highly potent, and selective orexin receptor 2 (OX2R) agonist.
ORX750 is designed to activate orexin signaling in the brain and treat the underlying cause of NT1.
ORX750 was designed by Centessa using structure-based drug design capabilities, high-resolution protein crystallography, and cryo-EM.
Novel MOA

At Centessa, we are exploring additional molecules for potential expansion opportunities into a range of sleep-wake disorders and broader neurological indications.
ORX750 is an investigational agent that has not been approved by the FDA or any other regulatory authority.
Ongoing Clinical Studies
ORX750 is an investigational agent that has not been approved by the FDA or any other regulatory authority.
ORX750 has not been administered as an investigational drug to humans in any jurisdiction.
NCTxxxxxxx: Lorem Ipsum
For additional detail, please visit www.clinicaltrials.gov (US), www.clinicaltrialsregister.eu (EU), or registries in other jurisdictions.